Could its deregulation be at the root of all human (non-genetic) diseases?

By Jean-Marc SABATIER and Ziad FAJLOUN*
What is the renin-angiotensin system?
The ubiquitous renin-angiotensin system (RAS), also known as the renin-angiotensin-aldosterone system, is a hormonal and physiological system whose function is to control blood pressure and circulating blood volume in humans. The RAS also regulates pulmonary, cardiovascular, vegetative and renal functions, innate immunity and various microbiota, including the intestinal microbiota. The RAS controls autophagy, a cellular regeneration process that eliminates waste and damaged elements within the cell (a process initiated after 3 days of fasting). It is a form of endocrine and enzymatic regulatory cascade. It is present in all the body’s organs and tissues. The RAS has endocrine, autocrine, paracrine and intracrine activity.
RAS components are found both on the plasma membrane of cells and on the membranes of various organelles, such as mitochondrial membranes.
Mechanism of action: a delicate balance
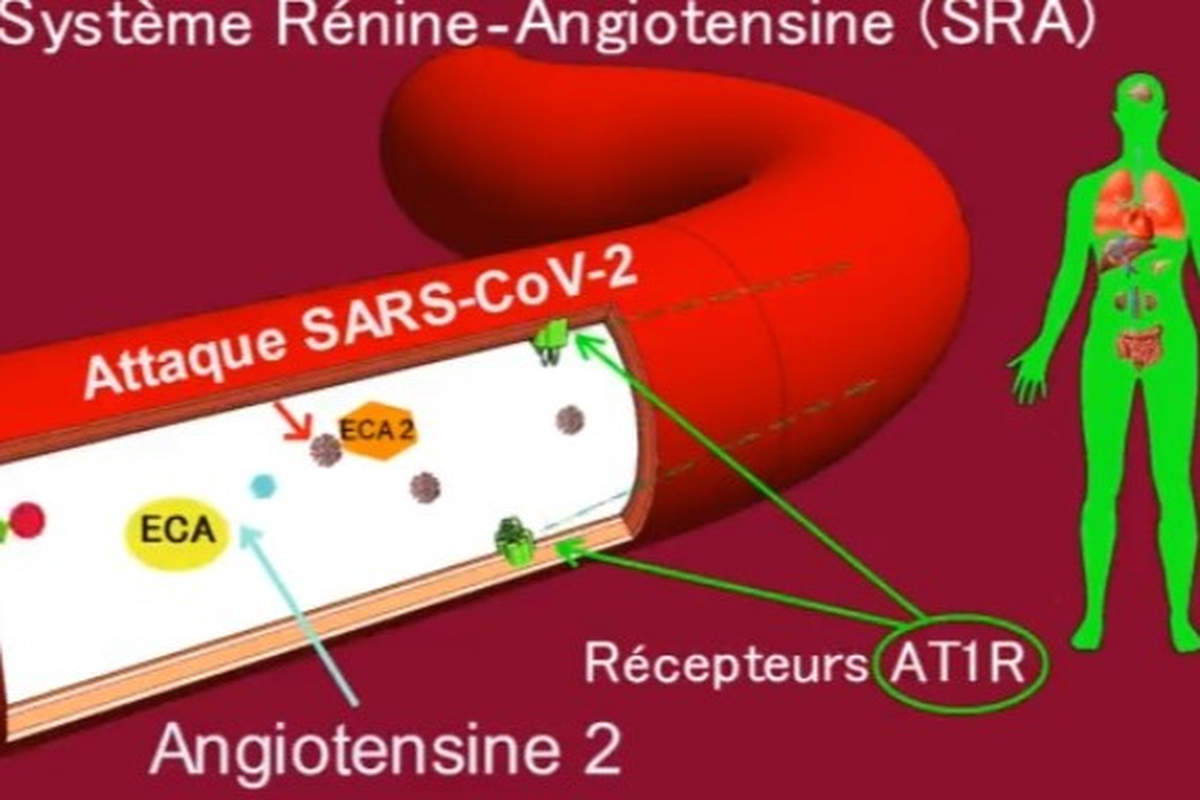
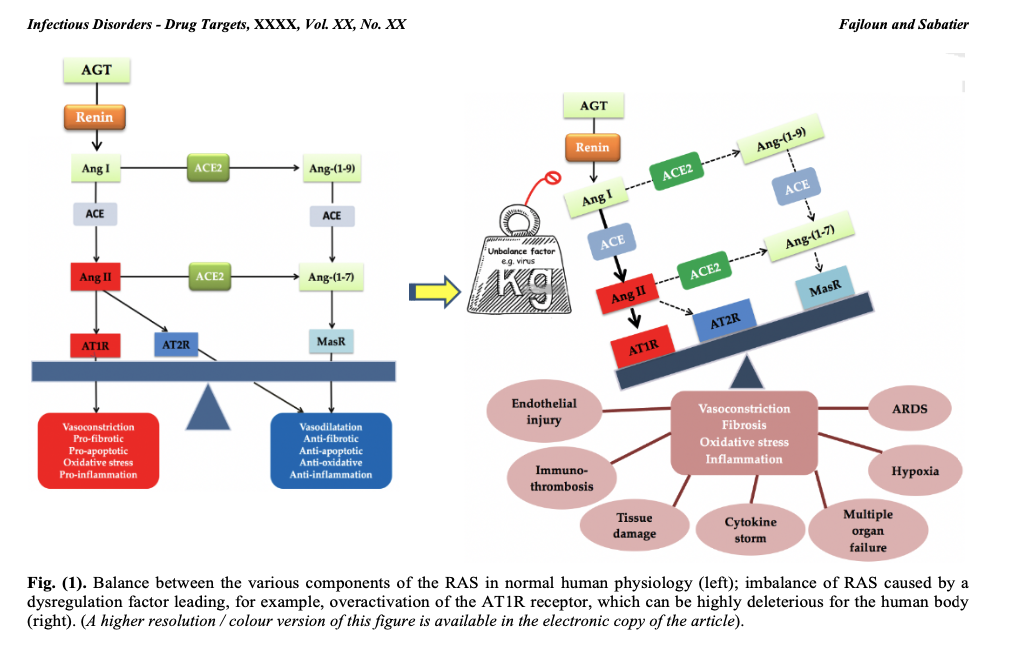
In the RAS, renin secreted by the kidney cleaves angiotensinogen (AGT) secreted by the liver to produce angiotensin I (Ang I). The latter is cleaved by angiotensin-converting enzyme (ACE1) to produce angiotensin II (Ang II). Ang II binds to receptors mediating vasoconstriction type 1 (AT1R) and mediating vasoconstriction type 2 (AT2R). Ang II is also cleaved by angiotensin-converting enzyme 2 (ACE2) to generate angiotensin (1-7) [Ang-(1-7)], which interacts with the G protein-coupled proto-oncogene Mas receptor (MasR) [7,8] and the AT2R receptor.
Ang II, by acting on the AT1R receptor, exerts several deleterious actions, such as vasoconstriction, fibrosis, apoptosis, thrombosis, hypoxemia, hypoxia, organ hypertrophy, angiogenesis, oxidative stress, inflammation and nitric oxide (NO) drop [9]. The enzyme ECA2 counterbalances the effects of Ang II/AT1R by cleaving Ang I and Ang II into Angiotensin (1-9) [Ang-(1-9)] and Ang-(1-7), respectively, favorably regulating the RAS.
Thus, a balance between the various components of the RAS must always be present to ensure proper functioning of the system and physiological regulation. On the other hand, an imbalance in the RAS can be highly deleterious to the human organism, as is the case with infection by the SARS-CoV-2 virus, which interacts with ACE2 (the SARS-CoV-2 receptor and a major RAS enzyme), leading to an imbalance in the RAS associated with overactivation of the AT1R receptor (see figure 1). COVID-19 symptoms will ultimately be linked to RAS dysfunction, as explained in our previously published work.
RAS dysregulation and human (non-genetic) diseases
In this article, we argued that RAS dysfunction is at the root of human (non-genetic) pathologies. Indeed, this goes far beyond COVID-19. To understand this, we need to take into account the genetic polymorphism of the actors (different components) of the RAS. Indeed, numerous studies have described strong links between the genetic polymorphism of RAS components and the prevalence of specific human diseases. We therefore believe that genetic factors may predispose the host to varying degrees of susceptibility to different human pathologies.
The prevalence and outcome of human diseases have been shown to be linked to genetic and protein polymorphisms of angiotensinogen, angiotensin-converting enzymes 1 and 2 (ACE1 and ACE2) and the AT1R receptor.
In fact, genetic polymorphism of RAS elements can alter their biological activities and expression levels, leading to increased capillary permeability, coagulation, fibrosis and apoptosis in alveolar and other cell types.
The Ang II/AT1R axis is the major pathway that can be affected during RAS deregulation. We have previously reported that the deleterious episodes following SARS-CoV-2 infection are the result of overactivation of the Ang II/AT1R axis.
Numerous deleterious effects can be produced by Ang II/AT1R signaling, such as vasoconstriction, fibrosis, inflammation, cell growth, migration, organ hypertrophy, thrombosis, production of reactive oxygen species (ROS) and more. Mitochondrial dysfunction, DNA damage and cytokine storms result from overactivation of the Ang II/AT1R axis. Dysregulated RAS components can also alter and induce an imbalance in the innate/acquired immune response, with an unusual predominance of hyper-stimulated macrophages, hyper-reactive mast cells and neutrophil granulocytes in affected human tissues.
For example, experiments have shown that deletion of the AT1R gene in rodents significantly improved vital functions and reduced oedema formation.
Furthermore, in the lung, the Ang II/AT1R axis increases vascular permeability by releasing prostaglandins and vascular endothelial growth factor, due to its pro-inflammatory, destructive and pro-fibrotic properties. Indeed, in the case of SARS coronavirus infection, for example, the virus invades the airways, vascular endothelial cells and epithelial cells, which will be severely damaged, triggering an accumulation of protein-rich edema fluid in the alveoli and pulmonary interstitium, activating macrophages and neutrophil granulocytes to release a wide range of inflammatory factors.
In the cardiovascular system, stimulation of the Ang II/AT1R axis has been associated with the development of various pathologies, including hypertension, vascular inflammation, atherosclerosis and heart failure.
In the nervous system, increased Ang II levels contribute to the loss of neurons in various brain regions. Ang II has been shown to cause the death of dopaminergic neurons, while “Losartan”, an AT1R receptor antagonist, protects these neurons from apoptosis.
In the digestive system, RAS has been shown to induce colonic inflammation by stimulating Th17 activation. In the colonic mucosa, Ang II induces colitis via AT1R via the JAK2/STAT1/3 pathway. In addition, regulation of the Ang II/AT1R axis is also involved in the control of dietary amino acid homeostasis (some of which are precursors of neurotransmitters such as serotonin, dopamine, adrenaline and noradrenaline, or other NO precursors), antimicrobial peptide expression and microbial flora including those of the lungs and intestines.
In the kidney, RAS hyperactivation has been described as playing a central role in the progression of chronic kidney disease. Indeed, the Ang II/AT1R axis induces fibrosis and inflammation, which appear to contribute to renal pathologies.
In short, overactivation of the Ang II/AT1R axis induces deleterious effects in humans (and mammals in general), as mentioned above. Nevertheless, there is a favorable regulatory system at RAS level involving players such as ligands Ang-(1-7), Ang-(1-9), Ang IV, alamandin, MasR receptors [10], AT2R, AT4R, MRGD, which via cell signaling, can produce anti-inflammatory, anti-fibrotic, anti-proliferative, anti-oxidant (reducing), anti-hypertensive, anti-hypertrophic, anti-angiogenic, anti-hypoxemic, anti-hypoxic and anti-thrombotic effects.
It should also be noted that the RAS evolves throughout life (the RAS of infants differs from that of children, adults and the elderly), from birth to death. The RAS also differs between men and women (the gene coding for the ECA2 receptor is carried on the X chromosome). Omnipresent in the body at organ, tissue and cell level, the RAS controls all functions related to cellular life.
This unprecedented global “vision” of the importance of the RAS in the total control of the functioning of the human body, as well as in the potential triggering of various neurological/neurodegenerative (dementia, Alzheimer’s and Parkinson’s diseases, schizophrenia, etc.), cardiovascular, gastrointestinal, immune (autoimmunity), cancerous and other pathologies, is unprecedented. It is “revolutionary” because it has never been suspected or described before.
Thus, we emphasize the extreme importance of the RAS, a hormonal system initially identified in the kidneys and liver, but ultimately present in all tissues and organs. It controls all the vital functions of the body, including brain and immune functions.
Ultimately, we argue that the RAS is the key to all non-genetic, and potentially genetic, human pathologies, as it also acts on DNA and its repair. For example, it controls telomerase activity, which lengthens the ends of chromosomes, thereby increasing life expectancy. The RAS is ubiquitous in the body: there are local variants in different tissues and organs (with the same receptors and ligands involved but in variable proportions and tissue distribution, adapted to the function of these tissues/organs) and cells (the RAS is found in the outer membrane of cells, but also in the membranes of the nucleus, mitochondria, endosomes, exosomes, lysosomes, and in the endoplasmic or sarcoplasmic reticulum). It acts as the central operator of the human body’s functions; it is not the brain that controls these functions, as it is itself driven by the RAS. In fact, the RAS controls the functions of neurons and other nervous system cell types such as oligodendrocytes, astrocytes and microglial cells.
The RAS is the “master” system of the human body, and therefore offers unsuspected therapeutic potential. In-depth study of the RAS and its “deleterious” receptor should lead to major advances in applied medicine in the decades to come, opening up an immense field of medically unexplored research.
Jean-Marc SABATIER and Ziad FAJLOUN
November 2023
(Association Internationale pour une médecine scientifique indépendante et bienveillante -AIMSIB)
*Jean-Marc Sabatier is Director of Research at the CNRS and holds a PhD in Cell Biology and Microbiology and an HDR in Biochemistry.
*Ziad Fajloun is a researcher at the University of Tripoli.